What Will I Be Asked To Do?
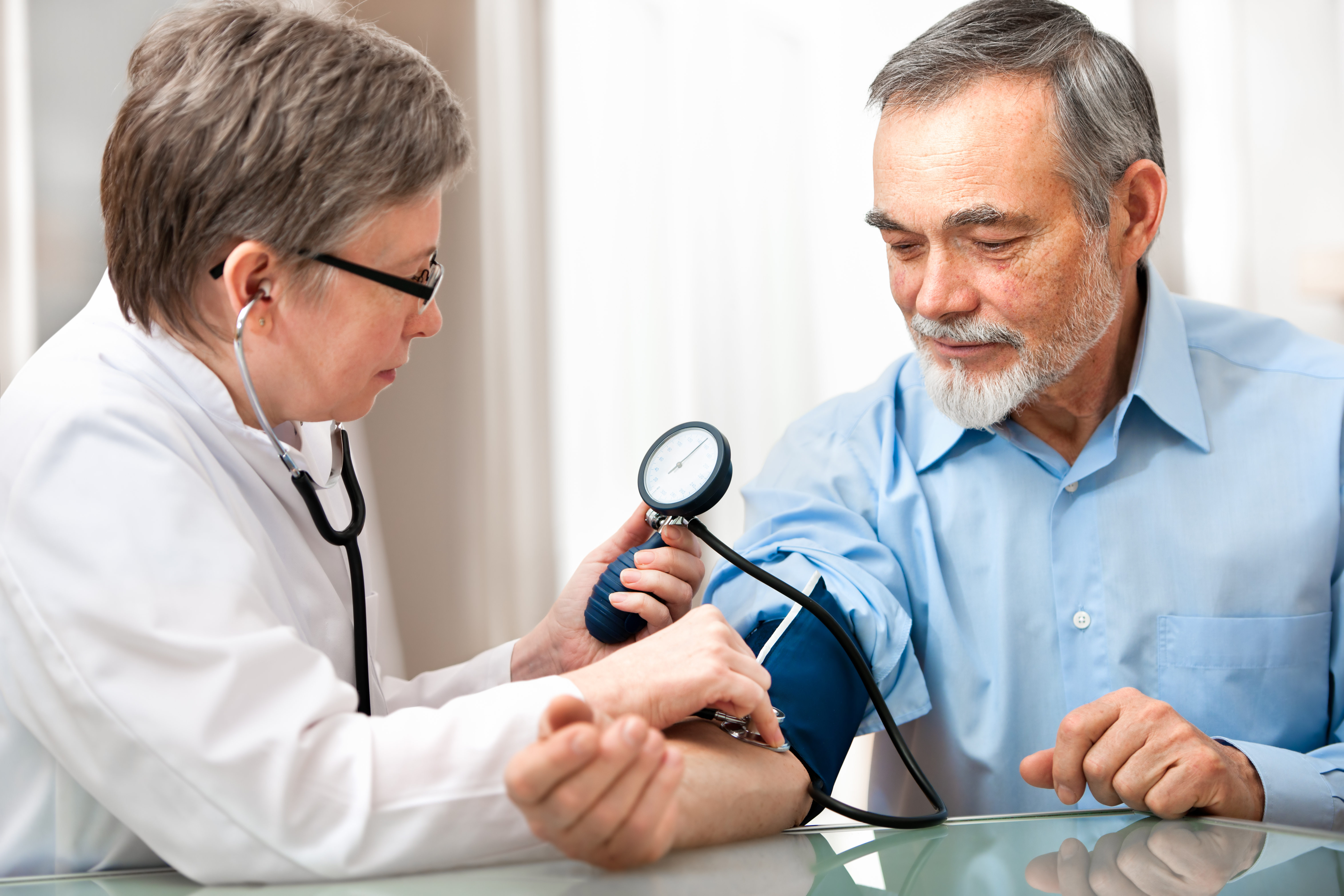
While you are in the study, you will be asked to attend study visits which will allow the study team to collect the information they need for the study, and more importantly, see how you are doing and monitor your health status. During these visits, the study team will:
- Discuss your past and current health and any medications you are taking and have taken in the recent past
- Ask how you are feeling and about any changes to your health since your last visit
- Conduct medical exams including measuring height, weight, and vital signs
- Perform other examinations, which may include electrocardiogram (often called ECG), computed tomography (CT) and magnetic resonance imaging (MRI) scans, ECOG performance status evaluation (ability to perform self-care activities) and biopsies
- Test your blood and urine
- Give you your study medication
- Ask if you are taking any new medications and complete study questionnaires
Your participation is voluntary, and you are free to withdraw at any time, without giving any reason and without your medical care or legal rights being affected.